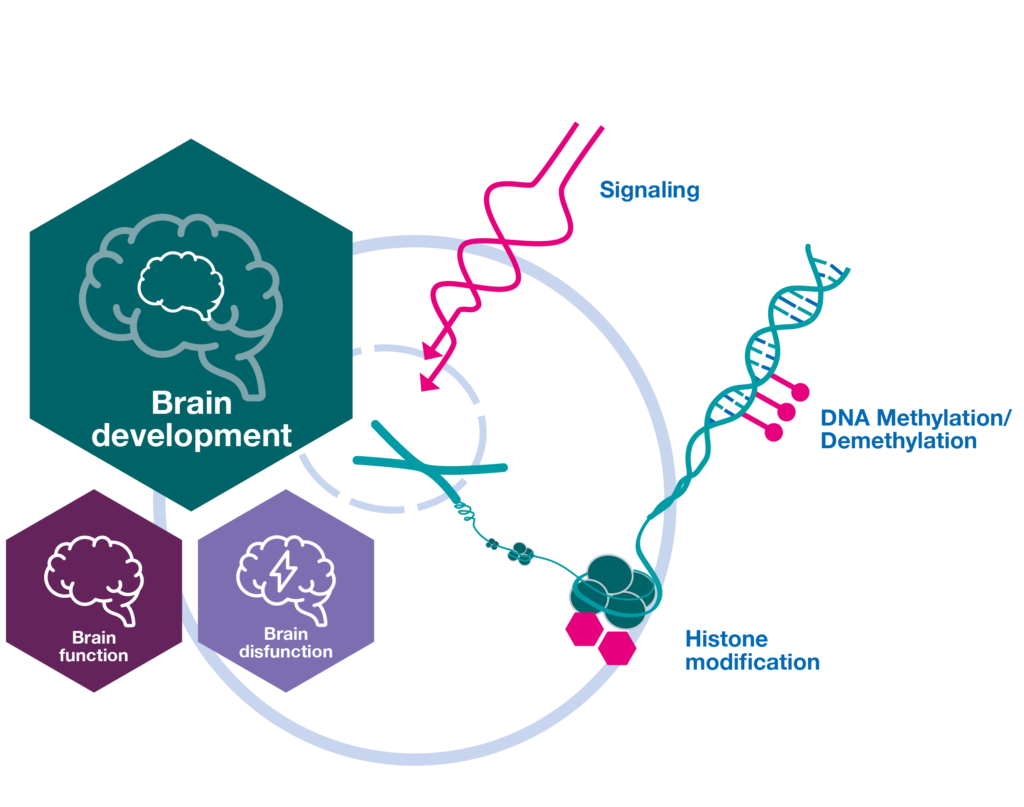
Adaptive neural epigenomes
Adaptation processes bearing a temporal component are the foundation of a developing organism and developmental processes often associate with changes in the epigenetic landscape.
It is therefore not surprising that epigenetic mechanisms are involved in different neurodevelopmental processes including control of proliferation and differentiation of neuronal stem cells, neuronal migration, cell survival, morphological maturation, and synapse formation/maturation.
Cell autonomous and non-autonomous communication pathways that impinge on epigenetic chromatin modification are far from being understood mechanistically and will be explored in SPP2502 projects.
Furthermore, recent research crystallizes that CNS diseases have an epigenetic layer, including neurodevelopmental diseases. Epigenetic processes not only affect neurodevelopmental disorders during development. But as a consequence of complex genome-environment interactions they leave traces surfacing in adulthood. A prime example is schizophrenia. The number of such diseases with a recognized epigenetic layer is raising steadily, further selected examples include epilepsy autism and depression.
Selected reading:
- Akol I, Izzo A, Gather F, Strack S, Heidrich S, Ó hAilín D, Villarreal A, Hacker C, Rauleac T, Bella C, Fischer A, Manke T, Vogel T. Multimodal epigenetic changes and altered NEUROD1 chromatin binding in the mouse hippocampus underlie FOXG1 syndrome. Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2122467120. doi: 10.1073/pnas.2122467120.
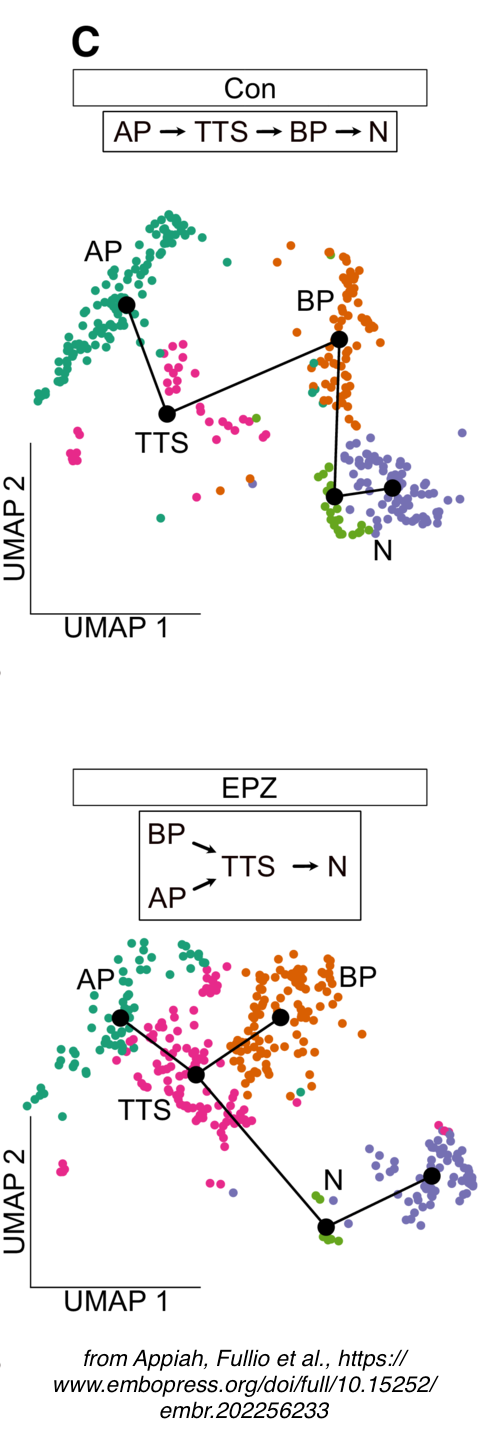
- Appiah B, Fullio CL, Ossola C, Bertani I, Restelli E, Cheffer A, Polenghi M, Haffner C, Garcia-Miralles M, Zeis P, Treppner M, Bovio P, Schlichtholz L, Mas-Sanchez A, Zografidou L, Winter J, Binder H, Grün D, Kalebic N, Taverna E, Vogel T. DOT1L activity affects neural stem cell division mode and reduces differentiation and ASNS expression. EMBO Rep. 2023 Aug 3;24(8):e56233. doi: 10.15252/embr.202256233.
- Cheffer A, Garcia-Miralles M, Maier E, Akol I, Franz H, Srinivasan VSV, Vogel T. DOT1L deletion impairs the development of cortical parvalbumin-expressing interneurons. Cereb Cortex. 2023 Sep 26;33(19):10272-10285. doi: 10.1093/cercor/bhad281.
- Bölicke N, Albert M. Polycomb-mediated gene regulation in human brain development and neurodevelopmental disorders. Dev Neurobiol. 2022 May;82(4):345-363. doi: 10.1002/dneu.22876.
- Michurina A, Sakib MS, Kerimoglu C, Kruger DM, Kaurani L, Islam MR, Joshi PD, Schroder S, Centeno TP, Zhou J, Pradhan R, Cha J, Xu X, Eichele G, Zeisberg EM, Kranz A, Stewart AF, Fischer A (2022) Postnatal expression of the lysine methyltransferase SETD1B is essential for learning and the regulation of neuron-enriched genes. EMBO J 41:e106459.
- Bachmann S, Linde J, Bell M, Spehr M, Zempel H, Zimmer-Bensch G. DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons. Int J Mol Sci. 2021 Feb 18;22(4):2034. doi: 10.3390/ijms22042034.
- Kerimoglu C, Pham L, Tonchev AB, Sakib MS, Xie Y, Sokpor G, Ulmke PA, Kaurani L, Abbas E, Nguyen H, Rosenbusch J, Michurina A, Capece V, Angelova M, Maricic N, Brand-Saberi B, Esgleas M, Albert M, Minkov R, Kovachev E, Teichmann U, Seong RH, Huttner WB, Nguyen HP, Stoykova A, Staiger JF, Fischer A, Tuoc T. H3 acetylation selectively promotes basal progenitor proliferation and neocortex expansion. Sci Adv. 2021 Sep 17;7(38):eabc6792. doi: 10.1126/sciadv.abc6792. Epub 2021 Sep 15. PMID: 34524839; PMCID: PMC8443185.
- Winick-Ng W, Kukalev A, Harabula I, Zea-Redondo L, Szabó D, Meijer M, Serebreni L, Zhang Y, Bianco S, Chiariello AM, Irastorza-Azcarate I, Thieme CJ, Sparks TM, Carvalho S, Fiorillo L, Musella F, Irani E, Torlai Triglia E, Kolodziejczyk AA, Abentung A, Apostolova G, Paul EJ, Franke V, Kempfer R, Akalin A, Teichmann SA, Dechant G, Ungless MA, Nicodemi M, Welch L, Castelo-Branco G, Pombo A. Cell-type specialization is encoded by specific chromatin topologies. Nature. 2021 Nov;599(7886):684-691. doi: 10.1038/s41586-021-04081-2. Epub 2021 Nov 17.
- Ferrari F, Arrigoni L, Franz H, Izzo A, Butenko L, Trompouki E, Vogel T, Manke T. DOT1L-mediated murine neuronal differentiation associates with H3K79me2 accumulation and preserves SOX2-enhancer accessibility. Nat Commun. 2020 Oct 15;11(1):5200. doi: 10.1038/s41467-020-19001-7.
- Gray de Cristoforis A, Ferrari F, Clotman F, Vogel T. Differentiation and localization of interneurons in the developing spinal cord depends on DOT1L expression. Mol Brain. 2020 May 29;13(1):85. doi: 10.1186/s13041-020-00623-3. PMID: 32471461.
- Pensold D, Reichard J, Van Loo KMJ, Ciganok N, Hahn A, Bayer C, Liebmann L, Groß J, Tittelmeier J, Lingner T, Salinas-Riester G, Symmank J, Halfmann C, González-Bermúdez L, Urbach A, Gehrmann J, Costa I, Pieler T, Hübner CA, Vatter H, Kampa B, Becker AJ, Zimmer-Bensch G. DNA Methylation-Mediated Modulation of Endocytosis as Potential Mechanism for Synaptic Function Regulation in Murine Inhibitory Cortical Interneurons. Cereb Cortex. 2020 Jun 1;30(7):3921-3937. doi: 10.1093/cercor/bhaa009.
- Symmank J, Bayer C, Reichard J, Pensold D, Zimmer-Bensch G. Neuronal Lhx1 expression is regulated by DNMT1-dependent modulation of histone marks. Epigenetics. 2020 Nov;15(11):1259-1274. doi: 10.1080/15592294.2020.1767372. Epub 2020 May 22.
- Franz H, Villarreal A, Heidrich S, Videm P, Kilpert F, Mestres I, Calegari F, Backofen R, Manke T, Vogel T. DOT1L promotes progenitor proliferation and primes neuronal layer identity in the developing cerebral cortex. Nucleic Acids Res. 2019 Jan 10;47(1):168-183. doi: 10.1093/nar/gky953.
- Urban I, Kerimoglu C, Sakib MS, Wang H, Benito E, Thaller C, Zhou X, Yan J, Fischer A, Eichele G. TIP60/KAT5 is required for neuronal viability in hippocampal CA1. Sci Rep. 2019 Nov 7;9(1):16173. doi: 10.1038/s41598-019-50927-1.
- Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, Islam MR, Capece V, Zhou Q, Edbauer D, Dean C, Fischer A. RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep. 2018 Apr 10;23(2):546-554. doi: 10.1016/j.celrep.2018.03.059.
- Symmank J, Bayer C, Schmidt C, Hahn A, Pensold D, Zimmer-Bensch G. DNMT1 modulates interneuron morphology by regulating Pak6 expression through crosstalk with histone modifications. Epigenetics. 2018;13(5):536-556. doi: 10.1080/15592294.2018.1475980.
- Zimmer-Bensch G. Diverse facets of cortical interneuron migration regulation – Implications of neuronal activity and epigenetics. Brain Res. 2018 Dec 1;1700:160-169. doi: 10.1016/j.brainres.2018.09.001.
- Albert M, Kalebic N, Florio M, Lakshmanaperumal N, Haffner C, Brandl H, Henry I, Huttner WB. Epigenome profiling and editing of neocortical progenitor cells during development. EMBO J. 2017 Sep 1;36(17):2642-2658. doi: 10.15252/embj.201796764.
- Bahari-Javan S, Varbanov H, Halder R, Benito E, Kaurani L, Burkhardt S, Anderson-Schmidt H, Anghelescu I, Budde M, Stilling RM, Costa J, Medina J, Dietrich DE, Figge C, Folkerts H, Gade K, Heilbronner U, Koller M, Konrad C, Nussbeck SY, Scherk H, Spitzer C, Stierl S, Stöckel J, Thiel A, von Hagen M, Zimmermann J, Zitzelsberger A, Schulz S, Schmitt A, Delalle I, Falkai P, Schulze TG, Dityatev A, Sananbenesi F, Fischer A. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc Natl Acad Sci U S A. 2017 Jun 6;114(23):E4686-E4694. doi: 10.1073/pnas.1613842114. Epub 2017 May 22. Erratum in: Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E6026. doi: 10.1073/pnas.1711340114.
- Ferrai C, Torlai Triglia E, Risner-Janiczek JR, Rito T, Rackham OJ, de Santiago I, Kukalev A, Nicodemi M, Akalin A, Li M, Ungless MA, Pombo A. RNA polymerase II primes Polycomb-repressed developmental genes throughout terminal neuronal differentiation. Mol Syst Biol. 2017 Oct 16;13(10):946. doi: 10.15252/msb.20177754.
- Grassi D, Franz H, Vezzali R, Bovio P, Heidrich S, Dehghanian F, Lagunas N, Belzung C, Krieglstein K, Vogel T. Neuronal Activity, TGFβ-Signaling and Unpredictable Chronic Stress Modulate Transcription of Gadd45 Family Members and DNA Methylation in the Hippocampus. Cereb Cortex. 2017 Aug 1;27(8):4166-4181. doi: 10.1093/cercor/bhx095.
- Kerimoglu C, Sakib MS, Jain G, Benito E, Burkhardt S, Capece V, Kaurani L, Halder R, Agís-Balboa RC, Stilling R, Urbanke H, Kranz A, Stewart AF, Fischer A. KMT2A and KMT2B Mediate Memory Function by Affecting Distinct Genomic Regions. Cell Rep. 2017 Jul 18;20(3):538-548. doi: 10.1016/j.celrep.2017.06.072.
- Pensold D, Symmank J, Hahn A, Lingner T, Salinas-Riester G, Downie BR, Ludewig F, Rotzsch A, Haag N, Andreas N, Schubert K, Hübner CA, Pieler T, Zimmer G. The DNA Methyltransferase 1 (DNMT1) Controls the Shape and Dynamics of Migrating POA-Derived Interneurons Fated for the Murine Cerebral Cortex. Cereb Cortex. 2017 Dec 1;27(12):5696-5714. doi: 10.1093/cercor/bhw341.
- Halder R, Hennion M, Vidal RO, Shomroni O, Rahman RU, Rajput A, Centeno TP, van Bebber F, Capece V, Garcia Vizcaino JC, Schuetz AL, Burkhardt S, Benito E, Navarro Sala M, Javan SB, Haass C, Schmid B, Fischer A, Bonn S. DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat Neurosci. 2016 Jan;19(1):102-10. doi: 10.1038/nn.4194.
- Roidl D, Hellbach N, Bovio PP, Villarreal A, Heidrich S, Nestel S, Grüning BA, Boenisch U, Vogel T. DOT1L Activity Promotes Proliferation and Protects Cortical Neural Stem Cells from Activation of ATF4-DDIT3-Mediated ER Stress In Vitro. Stem Cells. 2016 Jan;34(1):233-45. doi: 10.1002/stem.2187.
- Fischer A. Epigenetic memory: the Lamarckian brain. EMBO J. 2014 May 2;33(9):945-67. doi: 10.1002/embj.201387637. Epub 2014 Apr 9. PMID: 24719207; PMCID: PMC4193930.
- Stilling RM, Rönicke R, Benito E, Urbanke H, Capece V, Burkhardt S, Bahari-Javan S, Barth J, Sananbenesi F, Schütz AL, Dyczkowski J, Martinez-Hernandez A, Kerimoglu C, Dent SY, Bonn S, Reymann KG, Fischer A. K-Lysine acetyltransferase 2a regulates a hippocampal gene expression network linked to memory formation. EMBO J. 2014 Sep 1;33(17):1912-27. doi: 10.15252/embj.201487870.
- Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci. 2013 Feb 20;33(8):3452-64. doi: 10.1523/JNEUROSCI.3356-12.2013. Erratum in: J Neurosci. 2013 Apr 17;33(16):7108.
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010 May 7;328(5979):753-6. doi: 10.1126/science.1186088. Erratum in: Science. 2010 Jun 25;328(5986):1634.
- Albert M, Peters AH. Genetic and epigenetic control of early mouse development. Curr Opin Genet Dev. 2009 Apr;19(2):113-21. doi: 10.1016/j.gde.2009.03.004.
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007 May 10;447(7141):178-82. doi: 10.1038/nature05772.
Signals mediating epigenetic adaptation in neural genomes
Signals controlling or impacting the epigenetic machinery are likely, but largely unexplored. While signaling cascades stimulated by membrane receptors that interpret the local environment are well studied in CNS development, function and disease, we still face a surprising lack of knowledge about how such signaling events shape and adapt the epigenome.
Research integrating signaling and epigenetic mechanisms, decoding the up- and downstream molecular events of chromatin modification in neuronal development, provides the experimental basis for exploring the adaptive capacity of the brain, and at the same time advancing our understanding of neurodevelopmental diseases.
Selected reading:
- Goldberg M, Islam MR, Kerimoglu C, Lancelin C, Gisa V, Burkhardt S, Krüger DM, Marquardt T, Malchow B, Schmitt A, Falkai P, Sananbenesi F, Fischer A. Exercise as a model to identify microRNAs linked to human cognition: a role for microRNA-409 and microRNA-501. Transl Psychiatry. 2021 Oct 8;11(1):514. doi: 10.1038/s41398-021-01627-w.
- Islam MR, Lbik D, Sakib MS, Maximilian Hofmann R, Berulava T, Jiménez Mausbach M, Cha J, Goldberg M, Vakhtang E, Schiffmann C, Zieseniss A, Katschinski DM, Sananbenesi F, Toischer K, Fischer A. Epigenetic gene expression links heart failure to memory impairment. EMBO Mol Med. 2021 Mar 5;13(3):e11900. doi: 10.15252/emmm.201911900. Epub 2021 Jan 20.
- Pensold D, Gehrmann J, Pitschelatow G, Walberg A, Braunsteffer K, Reichard J, Ravaei A, Linde J, Lampert A, Costa IG, Zimmer-Bensch G. The Expression of the Cancer-Associated lncRNA Snhg15Is Modulated by EphrinA5-Induced Signaling. Int J Mol Sci. 2021 Jan 29;22(3):1332. doi: 10.3390/ijms22031332.
- Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, Islam MR, Capece V, Zhou Q, Edbauer D, Dean C, Fischer A. RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep. 2018 Apr 10;23(2):546-554. doi: 10.1016/j.celrep.2018.03.059.
- Zimmer-Bensch G. Diverse facets of cortical interneuron migration regulation – Implications of neuronal activity and epigenetics. Brain Res. 2018 Dec 1;1700:160-169. doi: 10.1016/j.brainres.2018.09.001.
- Bahari-Javan S, Varbanov H, Halder R, Benito E, Kaurani L, Burkhardt S, Anderson-Schmidt H, Anghelescu I, Budde M, Stilling RM, Costa J, Medina J, Dietrich DE, Figge C, Folkerts H, Gade K, Heilbronner U, Koller M, Konrad C, Nussbeck SY, Scherk H, Spitzer C, Stierl S, Stöckel J, Thiel A, von Hagen M, Zimmermann J, Zitzelsberger A, Schulz S, Schmitt A, Delalle I, Falkai P, Schulze TG, Dityatev A, Sananbenesi F, Fischer A. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc Natl Acad Sci U S A. 2017 Jun 6;114(23):E4686-E4694. doi: 10.1073/pnas.1613842114. Epub 2017 May 22. Erratum in: Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E6026. doi: 10.1073/pnas.1711340114.
- Fischer A. Environmental enrichment as a method to improve cognitive function. What can we learn from animal models? Neuroimage. 2016 May 1;131:42-7. doi: 10.1016/j.neuroimage.2015.11.039.
- Gerstmann K, Pensold D, Symmank J, Khundadze M, Hübner CA, Bolz J, Zimmer G. Thalamic afferents influence cortical progenitors via ephrin A5-EphA4 interactions. Development. 2015 Jan 1;142(1):140-50. doi: 10.1242/dev.104927.
- Wahane SD, Hellbach N, Prentzell MT, Weise SC, Vezzali R, Kreutz C, Timmer J, Krieglstein K, Thedieck K, Vogel T. PI3K-p110-alpha-subtype signalling mediates survival, proliferation and neurogenesis of cortical progenitor cells via activation of mTORC2. J Neurochem. 2014 Jul;130(2):255-67. doi: 10.1111/jnc.12718.
- Zimmer G, Rudolph J, Landmann J, Gerstmann K, Steinecke A, Gampe C, Bolz J. Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream. J Neurosci. 2011 Dec 14;31(50):18364-80. doi: 10.1523/JNEUROSCI.4690-11.2011.
- Vogel T, Stoykova A, Gruss P. Differential expression of polycomb repression complex 1 (PRC1) members in the developing mouse brain reveals multiple complexes. Dev Dyn. 2006 Sep;235(9):2574-85. doi: 10.1002/dvdy.20876.
- Zimmer G, Schanuel SM, Bürger S, Weth F, Steinecke A, Bolz J, Lent R. Chondroitin sulfate acts in concert with semaphorin 3A to guide tangential migration of cortical interneurons in the ventral telencephalon. Cereb Cortex. 2010 Oct;20(10):2411-22. doi: 10.1093/cercor/bhp309.
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007 May 10;447(7141):178-82. doi: 10.1038/nature05772.
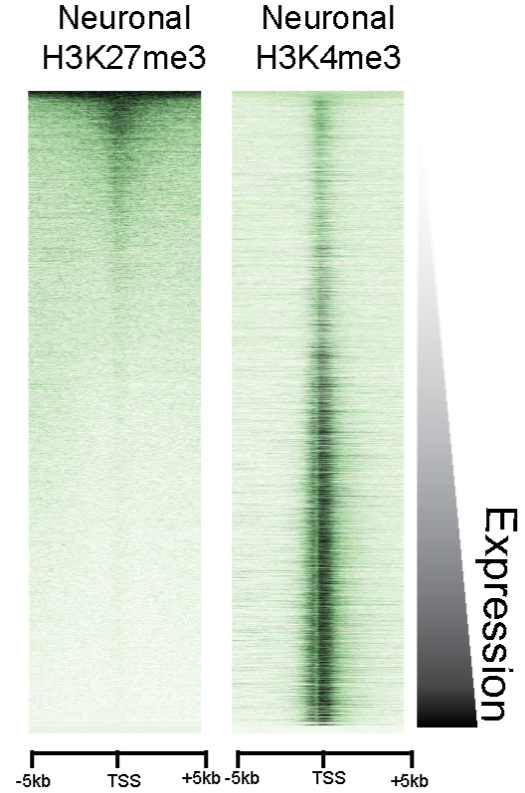
Technical advancements for studying epigenetics in neural cell transition and adaptation
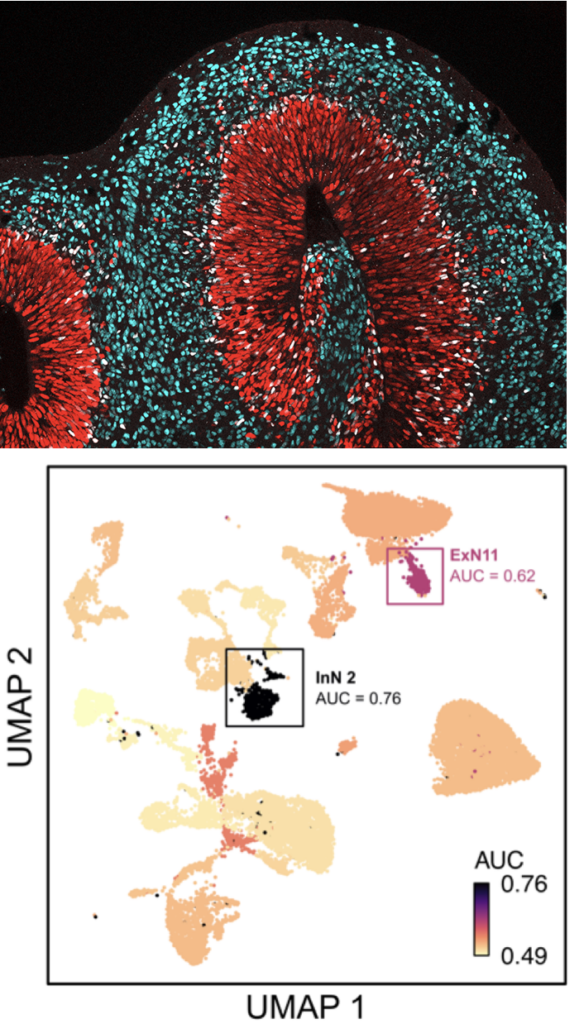
To address open mechanistic questions related to cell state transitions and adaptation processes in neuroepigenetics, the SPP2502 will integrate groups that advance and allow bioinformatics analyses. SPP2502 research will profit on recent technological advancements, especially in four different areas: (i) development of high throughput sequencing methods alongside respective bioinformatics tools, (ii) single-cell sequencing technology that combines transcriptomic and epigenomic analyses, (iii) genome-editing approaches conferring local viewpoint on epigenetic adaptation, and (iv) advanced cellular model systems that allow studying the human CNS, interactions in heterologous cellular systems, and employing reprogramming strategies.
Selected reading:
- Beagrie RA, Thieme CJ, Annunziatella C, Baugher C, Zhang Y, Schueler M, Kukalev A, Kempfer R, Chiariello AM, Bianco S, Li Y, Davis T, Scialdone A, Welch LR, Nicodemi M, Pombo A. Multiplex-GAM: genome-wide identification of chromatin contacts yields insights overlooked by Hi-C. Nat Methods. 2023 Jul;20(7):1037-1047. doi: 10.1038/s41592-023-01903-1.
- Ferrari F, Arrigoni L, Franz H, Izzo A, Butenko L, Trompouki E, Vogel T, Manke T. DOT1L-mediated murine neuronal differentiation associates with H3K79me2 accumulation and preserves SOX2-enhancer accessibility. Nat Commun. 2020 Oct 15;11(1):5200. doi: 10.1038/s41467-020-19001-7.
- Albert M, Kalebic N, Florio M, Lakshmanaperumal N, Haffner C, Brandl H, Henry I, Huttner WB. Epigenome profiling and editing of neocortical progenitor cells during development. EMBO J. 2017 Sep 1;36(17):2642-2658. doi: 10.15252/embj.201796764.
- Fraser J, Ferrai C, Chiariello AM, Schueler M, Rito T, Laudanno G, Barbieri M, Moore BL, Kraemer DC, Aitken S, Xie SQ, Morris KJ, Itoh M, Kawaji H, Jaeger I, Hayashizaki Y, Carninci P, Forrest AR; FANTOM Consortium; Semple CA, Dostie J, Pombo A, Nicodemi M. Hierarchical folding and reorganization of chromosomes are linked to transcriptional changes in cellular differentiation. Mol Syst Biol. 2015 Dec 23;11(12):852. doi: 10.15252/msb.20156492.